CONCLUSION
Hepatic light chain amyloidosis is caused by malignant plasma cells that abnormally secrete immunoglobulin light chains, which undergo a conformational change to a β-pleated sheet configuration and are deposited in the liver. The amyloid fibrils are deposited mainly in the hepatic sinusoids, the space of Disse and the walls of blood vessels. Previous autopsy studies have shown that the proportion of systemic light chain amyloidosis with liver involvement is extremely high; however, there are fewer systematic analyses of the clinical features in the Chinese population. Therefore, this study retrospectively analysed the clinical data of patients with hepatic lightchain amyloidosis in our center to further explore the clinical characteristics of this disease.
A total of 40 patients with hepatic light chain amyloidosis were included in the analysis from 2007 to 2022. Inclusion criteria were positive liver tissue biopsy or positive biopsy of other organs with alkaline phosphatase (AKP) greater than 1.5 times the upper limit of normal or total liver span >15 cm in the absence of heart failure.
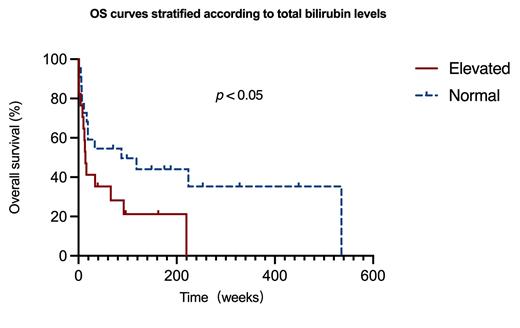
The median age of these patients was 58±10 years and 80% were male. Of these 40 patients, 66% had light-chain amyloidosis secondary to myeloma and the remainder had primary amyloidosis. The proportion of patients with symptoms related to liver injury as the initial clinical presentation was 33%. Symptoms included malaise, abdominal distention, or jaundice. 40% of patients with symptoms related to abnormal heart function, such as chest tightness, severe shortness of breath, and dizziness. 25% of the patients were first seen for symptoms related to impaired renal function, such as proteinuria with lower limb edema or hypourocrinia. κ-type was found in 28% of the patients and λ-type in 73%. All patients had other sites of involvement, 95% with cardiac involvement (72.9%, Mayo 2004 stage III) and 48% with renal involvement. AKP was elevated in 100% of patients, r-GT in 95%, liver enzymes in 33%, total bilirubin in 44%, and the maximum anteroposterior diameter of the right lobe of the liver was 16.6±1.7 cm (upper limit of normal was 10 cm). In this cohort of patients, 80% received a 2- to 3-drug combination regimen based on proteasome inhibitors and 5% received a 2-drug combination regimen based on melphalan, with a median survival time of 6.5±31.3 months, and patients with a normal total bilirubin level had an overall survival time 2.4 times longer than those with an elevated level (p< 0.05,Figure 1).
Contrary to previous reports of hepatic light chain amyloidosis, λ light chain amyloidosis was predominant in our center; elevated total bilirubin was an independent risk factor for survival.
Disclosures
No relevant conflicts of interest to declare.